PharmaSUG 2014 - Paper CC10
Automating the Number of Treatment Columns for a Dose Escalation Study
Catherine DeVerter, Novella Clinical, Morrisville, NC
Sonali Garg, Alexion Pharmaceuticals, Cheshire, CT
ABSTRACT
In dose escalation studies, there is the need to increase or decrease the number of treatment columns in table output
when a new treatment arm is added to the trial. If programs are not written to add the new columns dynamically, they
often need to be edited repeatedly. What if we can automate the number of columns to be displayed in the table
without having to go and edit the program?
This paper will show how SAS programmers can automate and control the number of columns in the table output
without having to change the PROC REPORT’s COLUMN and DEFINE statements again and again. The method
discussed in this paper will make use of the SQL procedure.
INTRODUCTION
In some early phase clinical trials (such as Phase I oncology), it is common to perform dose escalation studies to find
the most suitable dose for the drug being evaluated. Often a new dose is added as the trial progresses and outputting
all the columns for table output may turn into a nightmare if the change has to be done manually each time. To further
complicate things, sometimes the new dose is calculated as existing doses are evaluated (e.g., based on PK results),
so the exact doses evaluated in the trial may be not be known until statistical programming has already begun.
Macros can be written to have a treatment column outputted automatically for any new dose group that has been
entered into the clinical database so that TLF creation is automated.
DETERMINE TREATMENT VARIABLES WITH THE %TRTCOLS MACRO
The %TrtCols macro reads from the key subject level dataset (e.g., ADSL) to determine the different values that
currently exist for the treatment variable. The numeric treatment variable TRT01PN may be used, or any other
variable can be created that indicates the unique dose. For the purpose of this paper, we will use TRT01PN.
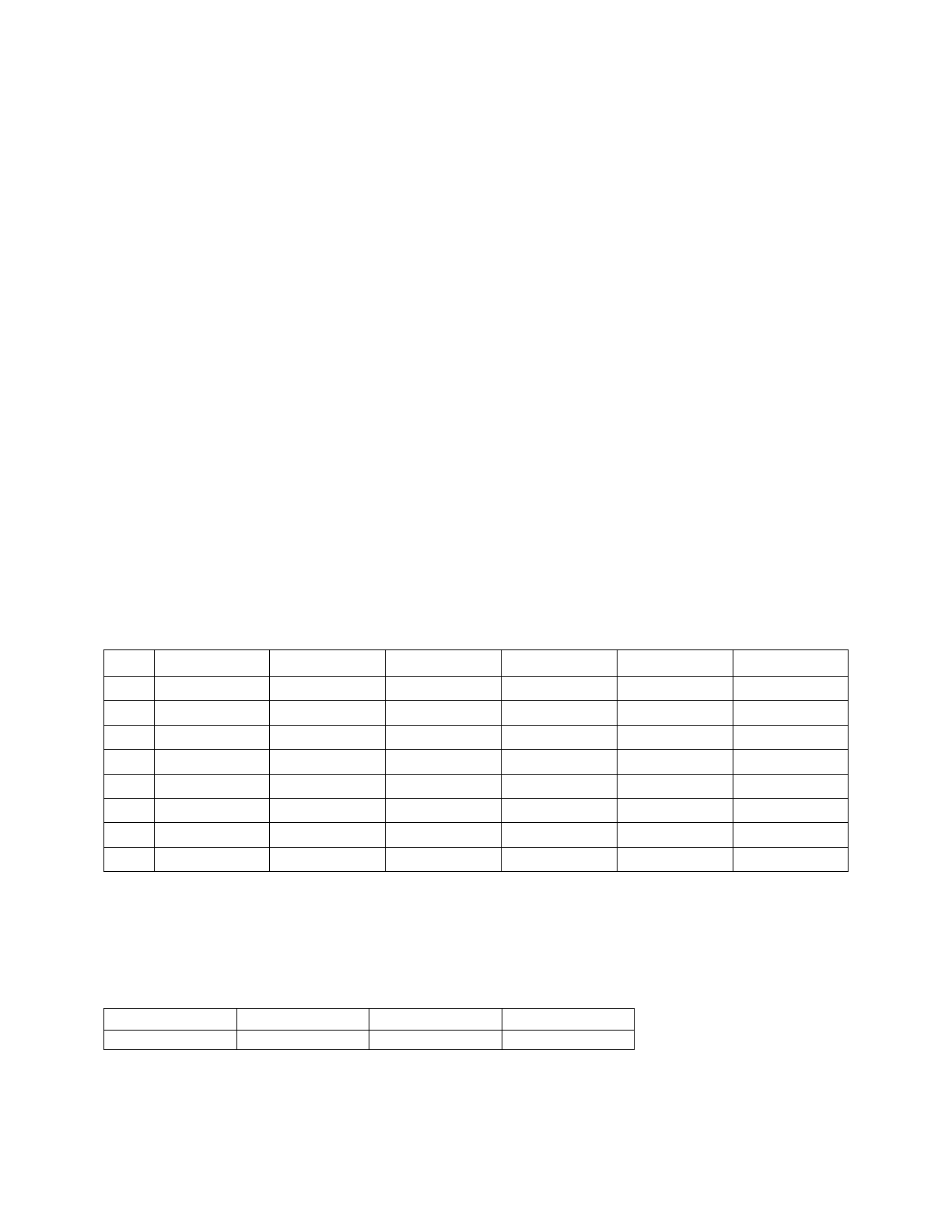
SUBJID
TRT01P
TRT01PN
ITTFL
SEX
AGE
1
01-101
10 mg
1
Y
M
25
2
01-102
10 mg
1
Y
M
36
3
01-103
50 mg
3
Y
F
41
4
01-103
50 mg
3
Y
M
35
5
01-104
25 mg
2
Y
F
22
6
01-106
25 mg
2
Y
F
50
7
01-108
10 mg
1
Y
M
48
8
01-110
25 mg
2
N
M
32
Table 1. Subject Level Dataset
The macro assumes that you have created a dataset called DENOM that has only one record and numeric variables
representing the N (i.e., number of subjects) of each treatment group. DENOM is a PROC FREQ of the treatment
variables of your subject level dataset that is then transposed with the new variables having a prefix of ‘COLUMN_’.
A variable for the overall total (or MTD/Maximum Tolerated Dose, if needed) can also be included. Table 2 displays
the DENOM dataset that results from the subject level dataset created for our example, restricted to subjects in the
ITT analysis population:
COLUMN_1
COLUMN_2
COLUMN_3
COLUMN_99
3
2
2
7
Table 2. DENOM Dataset
Note that DENOM contains one COLUMN_x variable for each value of TRT01PN along with COLUMN_99, which
represents the overall Total (i.e., All Subjects).

Automating the Number of Treatment Columns for a Dose Escalation Study, continued
2
INSIDE THE %TRTCOLS MACRO
%TrtCols uses the distinct values of TRT01PN to create a global macro variable COLS that is a string of variables
COLUMN_1-COLUMN_X along with a variable for the overall Total and/or MTD, if needed. Here, X denotes the value
of the assigned treatment. The resulting COLS variable will be included in the COLUMNS statement of PROC
REPORT.
The parameters used by %TrtCols are POP, to indicate the desired analysis population, and ALL, to designate a
value to use for the overall Total column.
%macro TrtCols(pop=ITTFL, all=99) ;
%global trttotcol cdesc nlist cols ;
%* Create dataset with analysis population counts for each treatment and the Total;
data adsl_ ;
set adata.adsl (where=(&pop='Y' and trt01pn>.z)) ;
output ;
%if &all^= %then %do ;
* Output for overall total ;
trt01pn=&all ;
output ;
%end ;
run ;
proc sql noprint nowarn ;
%** CDESC - Treatment labels (e.g., 10 mg, 20 mg, 50 mg) ;
%** COLS - List of treatment column variables (e.g., COLUMN_1) ;
%** NLIST - Number of treatment groups, including Total ;
select distinct
/* Get distinct values of TRT01P for treatment column labels */
case
when trt01pn=&all then 'Total'
else trt01p
end into :cdesc separated by ":"
from adsl_
order by trt01pn ;
select name into :cols separated by " "
from dictionary.columns
where libname='WORK' and memname="DENOM" and name like 'COLUMN%' ;
%let nlist=%sysfunc(countw(&cdesc,':')) ;
quit ;
%* Using DENOM, string the values together in the macro variable TRTN ;
data _null_ ;
set denom ;
length trtlist $100 ;
retain trtlist ' ' ;
%do i=1 %to &nlist ;
%let vname=%scan(&cols,&i,%str( )) ;
trtlist=catx(':', trtlist, strip(&vname)) ;
%if &i=&nlist %then call symput('trtn', trtlist) ;;
%end ;
run ;
%* Cycle through trt variables and assign to TRTxCOL macro variables, ;
%* where x=1, 2, ..., number of treatments. TRTTOTCOL is for the Total column. ;
%* These are the treatment column headers. ‘|’ is assumed to be the SPLIT ;
%* character used in PROC REPORT ;
%do z=1 %to &nlist ;
%let trtlbl=%scan(&cdesc,&z,:) ;

Automating the Number of Treatment Columns for a Dose Escalation Study, continued
3
%let trtpopn=%scan(&trtn,&z,:) ;
%if %scan(&trtlbl,1)^=Total %then %do ;
%global trt&z.col ;
%let trt&z.col=&trtlbl|(N=&trtpopn) ;
%end ;
%else %let trttotcol=&trtlbl|(N=&trtpopn) ;
%end ;
%mend TrtCols ;
The macro variables created are displayed in Output 1.
CDESC=10 mg:25 mg:50 mg:Total
COLS=COLUMN_1 COLUMN_2 COLUMN_3 COLUMN_99
NLIST=4
TRTN=3:2:2:7
TRT1COL=10 mg|(N=3)
TRT2COL=25 mg|(N=2)
TRT3COL=50 mg|(N=2)
TRTTOTCOL=Total|(N=7)
Output 1. Macro Variables Created from %TrtCols
GENERATING DEFINE STATEMENTS WITH THE %DEFSTMT MACRO
The %DefStmt macro dynamically creates the DEFINE statements that will be used in PROC REPORT for your table
output. It uses the SASHELP VCOLUMN view with PROC SQL to get the treatment variable names (COLUMN_1,
COLUMN_2, etc.) and references the COLS macro variable created in the %TrtCols macro.
INSIDE THE %DEFSTMT MACRO
%DefStmt assumes that the dataset containing your table data, represented by the macro parameter DSNAME, has
already been created and has the treatment columns in variables named COLUMN_1, COLUMN_2, etc. The other
parameters used by %DefStmt are JUST, to indicate justification for the column data values, and WIDTH, for the
column width.
The end result of %DefStmt is a global macro variable called DEFSTMT which is a string of DEFINE statements for
each treatment column.
%macro defStmt(dsname=, just=l, width=) ;
%global defstmt ;
data &dsname.0 (keep=&cols) ;
set &dsname ;
run ;
data &dsname.1 (KEEP=col name) ;
set sashelp.vcolumn (where=(memname="%upcase(&dsname.0)" )) end=eof ;
length col $3 ;
col=strip(put(_n_,best.)) ;
if eof then col="tot" ;
run ;
proc sql noprint ;
/* DEFINE statements for PROC REPORT */
select 'define ' || strip(name) || ' / %nrstr(%")&trt' ||strip(col)
||'col%nrstr(%") style(column)=[just=&just. cellwidth=&width]'
into :defstmt separated by '; '
from &dsname.1 ;
Automating the Number of Treatment Columns for a Dose Escalation Study, continued
4
quit ;
%let defstmt =&defstmt%nrstr(;);
%mend defStmt ;
The value of the resulting global DEFSTMT macro variable is displayed in Output 2.
define COLUMN_1 / "10 mg|(N=3)" style(column)=[just=l cellwidth=12%]; define
COLUMN_2 / "25 mg|(N=2)" style(column)=[just=l
cellwidth=12%]; define COLUMN_3 / "50 mg|(N=2)" style(column)=[just=l
cellwidth=12%]; define COLUMN_99 / "Total|(N=7)"
style(column)=[just=l cellwidth=12%];
Output 2. DEFINE statements created from %DefStmt
CALLING THE %TRTCOLS AND %DEFSTMT MACROS
The %TrtCols macro must be called prior to the %DefStmt macro, and both are called in the table program prior to
the PROC REPORT statement. The DEFSTMT macro variable created by %DefStmt is referenced inside PROC
REPORT, as shown below.
%TrtCols(pop=ITTFL, all=99) ;
%DefStmt(dsname=tabdata, width=12%) ;
ods rtf file="&out\Tables\T02.rtf" style=myStyle notoc_data ;
proc report data=tables.tabdata nowindows split='|' missing
style(report)=[width=100% cellpadding=0]
style(column)=[protectspecialchars=off asis=on just=l]
style(header)=[asis=on just=c] ;
column rowlbl &cols ;
define rowlbl / “Parameter” style(column)=[width=15%] style(header)=[just=l] ;
%unquote(&defstmt) ;
run ;
ods rtf close ;
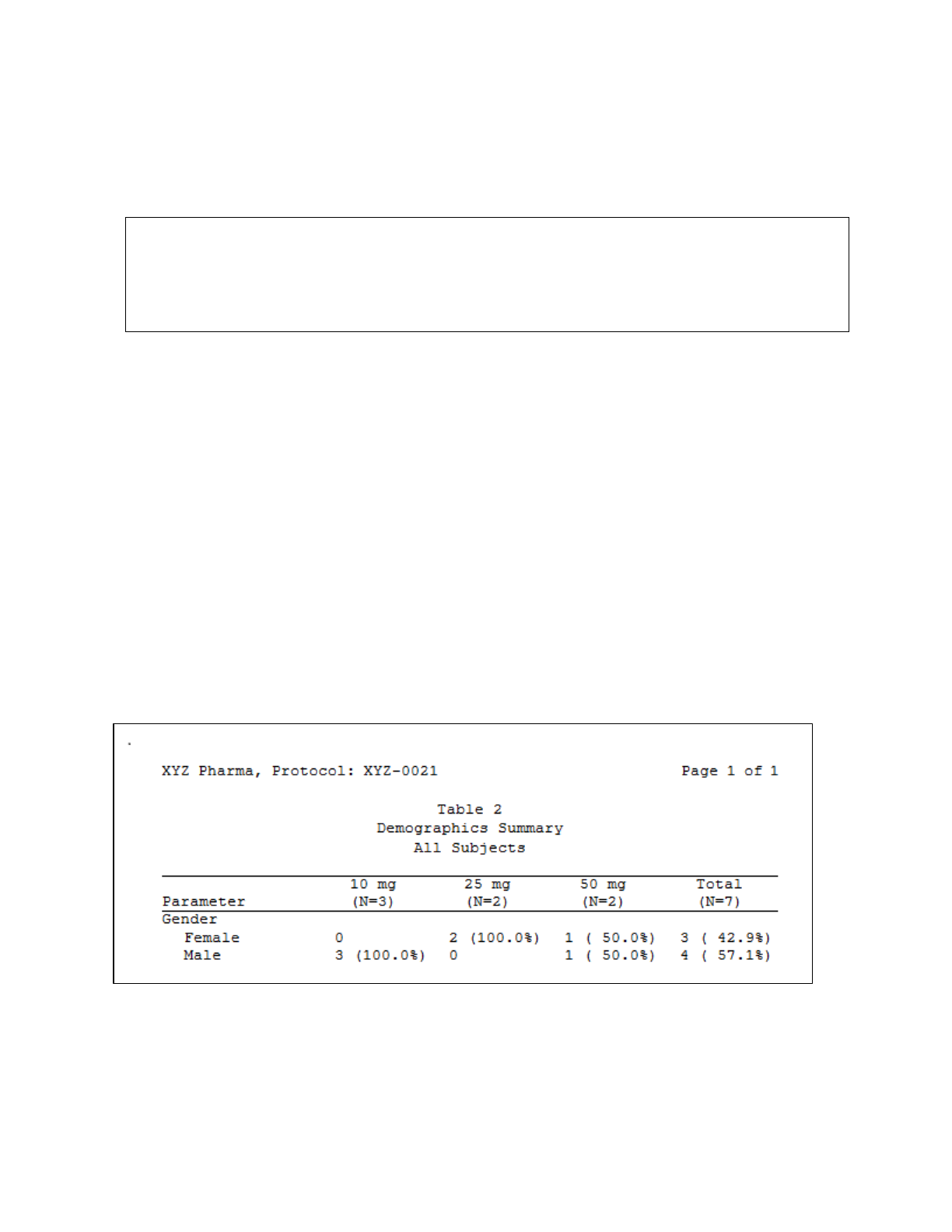
Finally, Display 1 demonstrates the output of the simple table created above using %TrtCols and %DefStmt.
Display 1. Table Output using Dynamically Created Treatment Column Headers

Automating the Number of Treatment Columns for a Dose Escalation Study, continued
5
LIMITATIONS/ASSUMPTIONS
• The code presented here works as is unless there are more than 5 or 6 treatment columns to display, depending
on column width. When there are more than that, formatting might behave unpredictably depending on the ODS
destination and options used. The example we have shown uses ODS RTF.
• %TrtCols and %DefStmt have to be called before PROC REPORT.
• The DEFINE statements and the treatment variables in the COLUMN statement of PROC REPORT will be listed
in the order corresponding to the TRT01PN value.
CONCLUSION
This paper demonstrates how we can automate the display of treatment columns in PROC REPORT when the
number of treatments is unknown or the actual treatment values are assigned during the course of the trial. The
method discussed takes advantage of SASHELP views, PROC SQL and the SAS Macro Language, and allows for
programming that will adapt dynamically to changing treatment groups without having to manually update the TLF
program whenever a new treatment group is added.
CONTACT INFORMATION
Your comments and questions are valued and encouraged. Contact the authors at:
Name: Catherine DeVerter
Enterprise: Novella Clinical
Address: 1700 Perimeter Park Drive
City, State ZIP: Morrisville, NC 27560
E-mail: cdeverter@novellaclinical.com
Name: Sonali Garg
Enterprise: Alexion Pharmaceuticals
Address: 352 Knotter Drive
City, State ZIP: Cheshire, CT 06410
E-mail: gargs@alxn.com
SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS
Institute Inc. in the USA and other countries. ® indicates USA registration.
Other brand and product names are trademarks of their respective companies.
